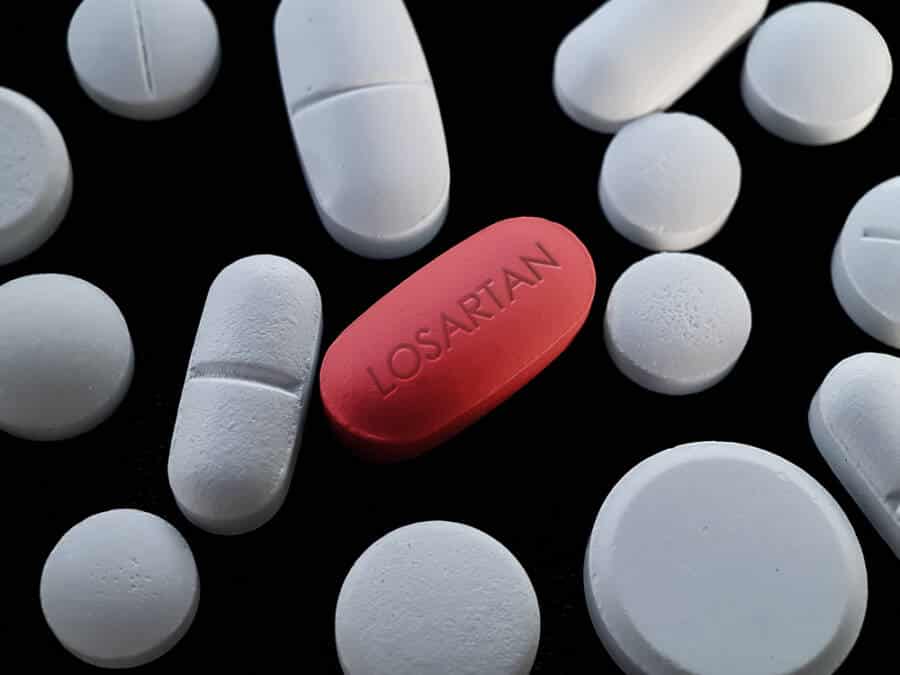
Losartan and Valsartan
Some types of blood pressure drugs have been recalled after scientists realized they contain NDMA. It was also established that these two were filled with carcinogens. The Food and Drug Administration recalled Losartan back in 2019.
According to Federal health experts, the drug had unacceptable levels of NMBA. The announcement came after a series of other blood pressure medications, after continuous proof that it contained the carcinogen.
The agency stated that it had identified traces of the carcinogen in Valsartan after being in the market for no less than four years. The FDA also estimates that there might be one more case above the average rate for every 8,000 people on the highest dose of the drug.






5 Responses
Maybe
TY for informing me about Losartan. Would you please email me your information about it separately? I could not copy it on my library’s computer and I must send it to my doctor.
So poorly written.
Does Duloxetine cause cancer?
Why are these drugs made this way are drug companies trying to kill the people.